abbott
ID NOW™
Isothermal PCR analyzerTHERE IS NO TIME LIKE ID NOW.
Formerly Alere™ i. New and Improved Speed, Performance and Efficiency. Plus a new name.
ID NOW™ is a rapid, instrument-based, isothermal system for the qualitative detection of infectious diseases. Our unique ID NOW™ isothermal nucleic acid amplification technology provides molecular results in just minutes, allowing you to make effective clinical decisions sooner.
ID NOW™ combines the benefits of speed and accuracy. You no longer need to compromise when testing your patients and making clinical decisions. A rapid molecular result with ID NOW™ means no longer having to choose.
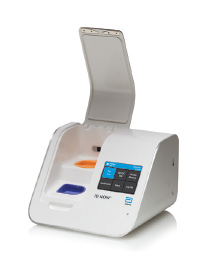
The intuitively designed ID NOW™ instrument can have a positive impact in any healthcare setting.
- Easy to use with only minimal training requirements
- Large visual touchscreen displays results, eliminating transcription errors and the need for printing
- Small footprint saves you bench space and can be used in any healthcare setting
- QC lock-out
- Bi-directional connectivity
- Offers exceptional performance
- Eliminates interpretation and transcription errors
- Gives you the confidence to make clinical decisions sooner
- Facilitates effective patient management
- Enables prompt initiation of infection control measures
- Aids targeted antiviral therapy and Antimicrobial Stewardship
- Reliable near-patient testing reduces overall healthcare costs
Formerly Alere™ i. The Fastest Rapid Molecular Flu Test. Plus a new name.
The ID NOW™ Influenza A & B 2 assay delivers molecular flu results in 13 minutes or less on our unique ID NOW™ platform; making it significantly faster than other molecular methods and more accurate than conventional rapid tests. Rapid diagnostic tests with increased sensitivity are essential for the reliable detection of influenza A and B and enable immediate, effective treatment decisions.
- Early detection – positive results in as few as 5 minutes, with 95% detected within 7 minutes
- Negative results in 13 minutes
- Detects up to 42% more true positives than RADTs3
- Room temperature storage
- CLIA waived
- Preferred sample type4
- Gives you the confidence to make clinical decisions sooner
- Facilitates effective patient management
- Aids targeted antiviral therapy and Antimicrobial Stewardship
ID NOW™ technology
Learn how Abbott utilizes unique isothermal nucleic acid amplification technology.* For more information about ID NOW™ Click Here
